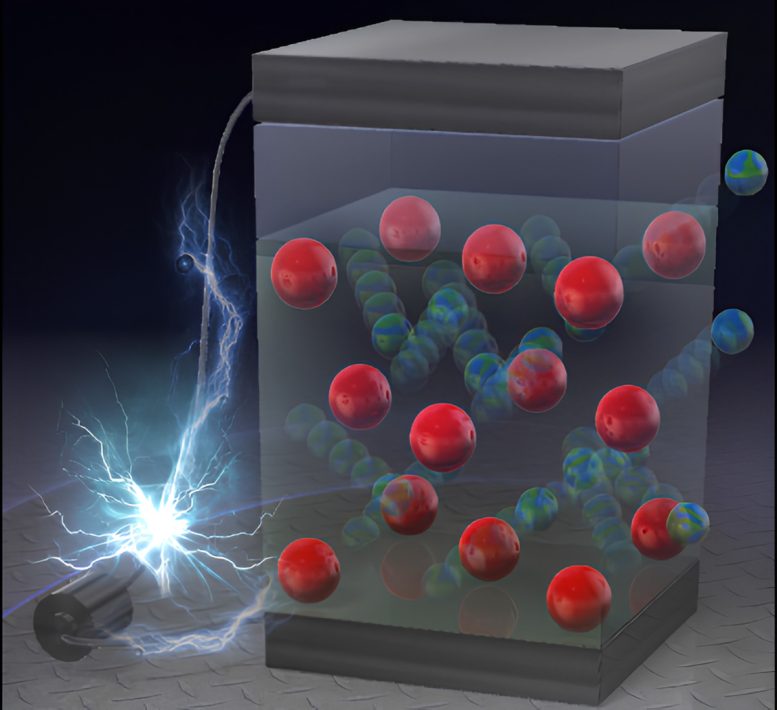
Enhancements in lithium-ion battery know-how have been achieved by introducing ample parts into the cathode materials, bettering power capability and stability, and decreasing environmental affect, paving the way in which for future commercialization. Credit score: Science Graphics. Co., Ltd.
Researchers have considerably improved the efficiency of lithium-iron-oxide cathodes utilized in lithium-ion batteries by doping them with ample parts like aluminum and silicon.
Cost-recharge biking of lithium-superrich iron oxide, an economical and high-capacity cathode for new-generation lithium-ion batteries, could be enormously improved by doping with available mineral parts.
The power capability and charge-recharge biking (cyclability) of lithium-iron-oxide, an economical cathode materials for rechargeable lithium-ion batteries, is improved by including small quantities of ample parts. The event, achieved by researchers at Hokkaido University, Tohoku College, and Nagoya Institute of Expertise, is reported within the journal ACS Supplies Letters.
Lithium-ion batteries have turn into indispensable in fashionable life, utilized in a large number of purposes together with cell phones, electrical autos, and enormous energy storage programs. A continuing analysis effort is underway to extend their capability, effectivity, and sustainability. A serious problem is to scale back the reliance on uncommon and costly sources. One strategy is to make use of extra environment friendly and sustainable supplies for the battery cathodes, the place key electron trade processes happen.
Analysis Developments and Challenges
The researchers labored to enhance the efficiency of cathodes primarily based on a specific lithium-iron-oxide compound. In 2023, they reported a promising cathode material, Li5FeO4, that reveals a excessive capability utilizing iron and oxygen redox reactions. Nevertheless, its improvement encountered issues related to the manufacturing of oxygen throughout charging-recharging biking.
“We’ve got now discovered that the cyclability could possibly be considerably enhanced by doping small quantities of abundantly accessible parts reminiscent of aluminum, silicon, phosphorus, and sulfur into the cathode’s crystal construction,” says Affiliate Professor Hiroaki Kobayashi on the Division of Chemistry, College of Science, Hokkaido College.
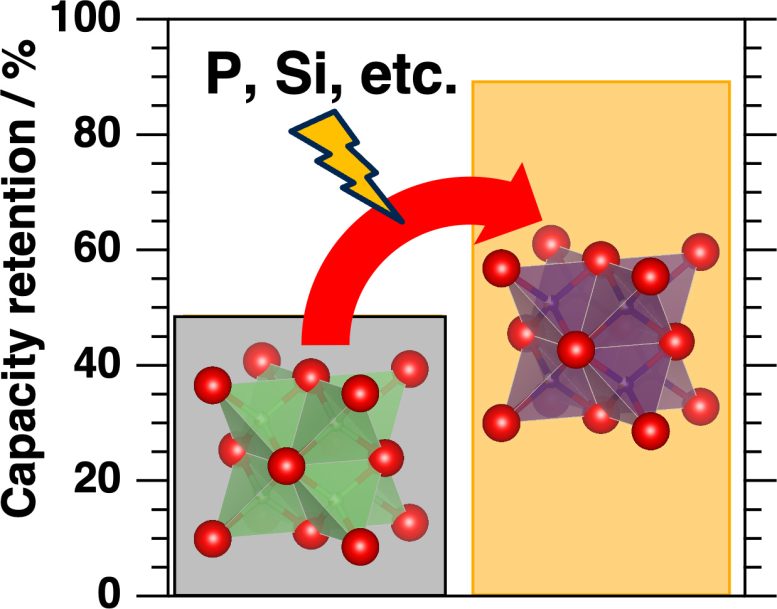
Capability retention of lithium-iron-oxide cathode is improved from 50% to 90% when doped with abundantly accessible parts reminiscent of aluminum, silicon, phosphorus, and sulfur. Credit score: Hiroaki Kobayashi
An important chemical facet of the enhancement proved to be the formation of robust ‘covalent’ bonds between the dopant and oxygen atoms inside the construction. These bonds maintain atoms collectively when electrons are shared between the atoms, quite than the ‘ionic’ interplay between constructive and negatively charged ions.
“The covalent bonding between the dopant and oxygen atoms makes the problematic launch of oxygen much less energetically favorable, and subsequently much less more likely to happen,” says Kobayashi.
The researchers used X-ray absorption evaluation and theoretical calculations to discover the high quality particulars of adjustments within the construction of the cathode materials brought on by introducing completely different dopant parts. This allowed them to suggest theoretical explanations for the enhancements they noticed. In addition they used electrochemical evaluation to quantify the enhancements within the cathode’s power capability, stability, and the biking between charging and discharging phases, displaying a rise in capability retention from 50% to 90%.
“We’ll proceed to develop these new insights, hoping to make a big contribution to the advances in battery know-how that might be essential if electrical energy is to extensively change fossil gasoline use, as required by international efforts to fight local weather change,” Kobayashi concludes.
The subsequent section of the analysis will embody exploring the challenges and prospects in scaling up the strategies into know-how prepared for commercialization.
Reference: “Towards Price-Efficient Excessive-Vitality Lithium-Ion Battery Cathodes: Covalent Bond Formation Empowers Stable-State Oxygen Redox in Antifluorite-Kind Lithium-Wealthy Iron Oxide” by Hiroaki Kobayashi, Yuki Nakamura, Yumika Yokoyama, Itaru Honma and Masanobu Nakayama, 22 April 2024, ACS Supplies Letters.
DOI: 10.1021/acsmaterialslett.4c00268