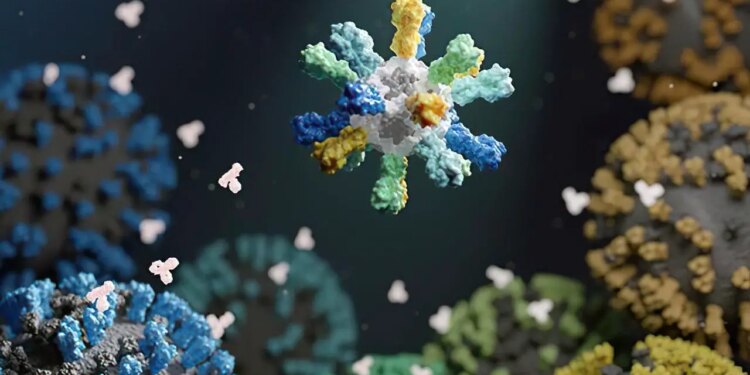
Illustration of a nanoparticle vaccine that comprises proteins from many various flu strains. Credit score: UW Medication Institute for Protein Design
The worldwide panorama underwent a sudden transformation in March 2020, when nations worldwide imposed lockdown measures to curb the swift proliferation of COVID-19. This often deadly disease, caused by a previously unknown coronavirus called SARS-CoV-2, sparked pandemics globally at an unprecedented pace. By the end of 2022, it had claimed the lives of over 6 million individuals globally.
However, COVID-19 wasn’t the first pandemic to besiege humanity. Merely 15 years prior, a pandemic influenza outbreak affected 60 million individuals worldwide. In 2003, an emergent disease known as severe acute respiratory syndrome, or SARS, infected over 8,000 people globally. This was triggered by a virus identified as the SARS-associated coronavirus (SARS-CoV). No one can predict when the next pandemic will happen—only that one eventually will.
These recent pandemics have brought into stark relief the need to be prepared for the next emerging disease, whenever it arrives. To this end, NIH-funded research teams have been working to develop universal vaccines against diseases with pandemic potential. Unlike current vaccines, which confer immunity to one or several strains of a disease, universal vaccines are designed to teach the immune system to defend against all versions of a pathogen—even versions that don’t exist yet. They do this by targeting an element of the pathogen that remains the same across all strains and types.
Such targets are usually those that are least accessible to the immune system. This has posed a significant challenge to vaccine researchers. But with recent progress in vaccine technology, researchers believe that universal vaccines are closer to reality than ever before.
Moving beyond educated guesses
For some viruses, the only constant is change. Locked in a continuous battle with the human immune system, many common viruses change, or mutate, rapidly. This means that even if you’ve been infected with a previous version of a virus, your immune system may not recognize an altered version the next time around.
A well-known example of the arms race between viruses and humans is the influenza virus, commonly known as the flu. More than 20 types of the virus—each of which, in turn, contains many different strains—circulate among people and animals, changing almost constantly.
The flu vaccine you get every year targets four strains that the scientific community predicts are most likely to predominate that season. “We have a well-established system to collect [information] on which strains are circulating all around the world,” says NIH vaccine researcher Dr. Karin Bok. “However it takes at the very least six months from the choice of which [strains] to incorporate to the vaccine being accessible to the general public.” And which flu strains flow into throughout that point can change unpredictably.
Because of this, seasonal flu vaccines range of their effectiveness. Their skill to stop extreme illness ranges from as excessive as 60% to as little as 10%.
All broadly used flu vaccines thus far educate the immune system to acknowledge a protein referred to as hemagglutinin, which is discovered on the floor of the influenza virus. The virus makes use of hemagglutinin to enter human cells.

A nanoparticle vaccine was made utilizing 60 randomly positioned receptor binding domains from eight totally different coronaviruses. Credit score: Wellcome Leap, Caltech, Merkin Institute
In a current NIH-funded examine, researchers designed a flu vaccine to supply broad safety in opposition to totally different influenza viruses. To create the vaccine, the researchers fused hemagglutinin to constructing blocks that assemble into nanometer-sized particles, or nanoparticles. The nanoparticles included hemagglutinin from 4 totally different flu strains. The researchers reasoned that this is able to encourage the immune system to answer elements of the protein that had been extra comparable, or conserved, between influenza strains.
“What these nanoparticles do is repetitively show the antigen—the protein from the virus—that you just’re attempting to mount an immune response to,” explains Dr. Neil King of UW Medication, who helped lead the examine together with researchers from NIH’s Vaccine Analysis Middle (VRC). “And repetition…tells the immune system that that is one thing harmful.” This method can create a robust immune reminiscence of the conserved a part of these viral proteins.
In research in mice, ferrets, and monkeys, the nanoparticle vaccines induced antibody responses in opposition to the included strains that had been pretty much as good as or higher than these elicited by a business vaccine. Notably, the nanoparticle vaccines additionally offered near-complete safety in opposition to a number of associated flu strains that weren’t included within the nanoparticles. In distinction, the business vaccine didn’t defend in opposition to these different strains.
“These nanoparticle vaccines could also be what we name a “supra-seasonal vaccine”—a vaccine that protects for multiple 12 months,” King says.
The vaccine, referred to as FluMos-v1, is now in phase 1 clinical trials.
VRC researchers have additionally been engaged on one other vaccine which will provoke a fair broader immune response to influenza. The group primarily based their method on the construction of hemagglutinin, which consists of a stem and a head. Flu vaccines thus far have focused the top of the protein, which is most accessible to immune cells. However that is additionally the a part of the protein that mutates quickest.
The brand new VRC vaccine, which has accomplished an early-phase human trial, makes use of a nanoparticle to show the hemagglutinin stem with out the top. The hemagglutinin stem tends to stay comparatively unchanged, at the same time as the top quickly modifications. The trial discovered that immunization with this vaccine was secure and elicited immune responses to a variety of hemagglutinins that lasted greater than a 12 months after vaccination.
Displaying the immune system the stem has an added benefit, Bok explains: it’s not one thing the immune system is used to seeing. This novelty provokes a stronger immune response. That, plus the conserved nature of the stem, “could make it so you’ll be capable of mount an immune response to any hemagglutinin you’re uncovered to [after vaccination],” Bok says.
A urgent want for common safety
The nanoparticle applied sciences pioneered to develop common flu vaccines at the moment are being examined to create vaccines that would defend in opposition to a number of present and future coronaviruses, together with SARS-CoV-2.
“SARS-CoV-2 has confirmed itself able to making new variants which are prolonging the worldwide COVID-19 pandemic,” says Dr. Pamela Bjorkman, who leads an NIH-funded analysis group on the California Institute of Expertise. And SARS-CoV-2 wasn’t the primary virus of its form—a sort referred to as a betacoronavirus—to leap from animals to individuals. SARS-CoV got here earlier than, and so did MERS-CoV, which causes the lethal Center East Respiratory Syndrome (MERS).
“The truth that three betacoronaviruses—SARS-CoV, MERS-CoV, and SARS-CoV-2—have spilled over into people from animal hosts within the final 20 years illustrates the necessity for making broadly protecting vaccines,” she says.
In a current examine, Bjorkman and her group mixed items of the spike proteins from eight totally different coronaviruses into a brand new nanoparticle vaccine. The portion of the spike protein they used known as the receptor binding area, or RBD. Coronaviruses use the RBD to enter human cells.
Every nanoparticle included 60 RBDs, in order that any two adjoining ones had been not often from the identical coronavirus. As with flu vaccines, this association encourages antibody-producing immune cells to focus on areas which are comparable throughout the proteins.
The group examined the brand new vaccine in mice engineered to be susceptible to SARS-CoV-2. Following vaccination, the mice produced antibodies that acknowledged a variety of various coronaviruses. And as anticipated, the antibodies acknowledged elements of the spike protein that remained comparable between coronaviruses.
Promising outcomes had been additionally seen when the vaccine was examined in monkeys. The animals had been protected not solely in opposition to a SARS-CoV-2 variant that wasn’t included within the vaccine but in addition in opposition to SARS-CoV.
“We will’t predict which virus or viruses among the many huge numbers in animals will evolve sooner or later to contaminate people to trigger one other epidemic or pandemic,” Bjorkman explains. “What we’re attempting to do is make an all-in-one vaccine protecting in opposition to SARS-like coronaviruses. This kind of vaccine would additionally defend in opposition to present and future SARS-CoV-2 variants with out the necessity for updating.”
The arrival of mRNA vaccines
One other software being examined to create extra broadly efficient vaccines is mRNA expertise. This expertise enabled COVID-19 vaccines to be developed and dropped at the clinic inside lower than a 12 months after the genome of SARS-CoV-2 was sequenced.
Historically, vaccines used weakened or killed variations of an precise pathogen, Bok explains. As expertise improved, extra refined vaccines had been made that included solely the pathogen proteins that work together with human cells. mRNA vaccines are very comparable, Bok says.
“You’re nonetheless getting the identical protein,” she explains. “It’s simply the supply mechanism that’s totally different. As an alternative of supplying you with the protein, it’s giving [your body] the supply code—the software program—so you may make [that protein] your self.”
This method permits the immune system to be uncovered to considerably higher portions of a protein than with a conventional vaccine. That, in flip, can produce a stronger immune response. One other benefit of mRNA vaccines is that they are often less expensive to supply and simpler to switch rapidly. These vaccines at the moment are being examined to stop quite a lot of diseases past SARS-CoV-2, together with influenza.
An NIH-funded analysis group led by Dr. Scott Hensley from the College of Pennsylvania designed a vaccine that features mRNAs for hemagglutinin from all 20 influenza sorts identified to contaminate individuals. It hadn’t been potential to incorporate a lot variation with conventional vaccine manufacturing strategies. However the researchers thought it would work with mRNA expertise.
In animal assessments, mice that acquired the experimental mRNA vaccine produced antibodies in opposition to each comparable and distinctive areas of all 20 several types of hemagglutinin. Ranges of those antibodies remained unchanged for months after vaccination. This sturdy antibody manufacturing occurred whether or not or not the mice had beforehand been uncovered to one of many flu strains.
Additional experiments confirmed that vaccination protected each mice and ferrets from a harmful flu pressure much like a type of within the vaccine.
“For a traditional vaccine, immunizing in opposition to all these sorts could be a significant problem, however with mRNA expertise it’s comparatively straightforward,” Hensley says. “The thought right here is to have a vaccine that can give individuals a baseline degree of immune reminiscence to various flu strains, in order that there will likely be far much less illness and dying when the subsequent flu pandemic happens.”
“Once we take into consideration pandemic preparedness,” Bok says, “what we’re most nervous about is the primary few months, earlier than vaccines may be ready. Common vaccines, together with antivirals and different therapies, may present [vital] safety in opposition to extreme illness from the subsequent pandemic.”
References: “Quadrivalent influenza nanoparticle vaccines induce broad safety” by Seyhan Boyoglu-Barnum, Daniel Ellis, Rebecca A. Gillespie, Geoffrey B. Hutchinson, Younger-Jun Park, Syed M. Moin, Oliver J. Acton, Rashmi Ravichandran, Mike Murphy, Deleah Pettie, Nick Matheson, Lauren Carter, Adrian Creanga, Michael J. Watson, Sally Kephart, Sila Ataca, John R. Vaile, George Ueda, Michelle C. Crank, Lance Stewart, Kelly Okay. Lee, Miklos Guttman, David Baker, John R. Mascola, David Veesler, Barney S. Graham, Neil P. King and Masaru Kanekiyo, 24 March 2021, Nature.
DOI: 10.1038/s41586-021-03365-x
“A multivalent nucleoside-modified mRNA vaccine in opposition to all identified influenza virus subtypes” by Claudia P. Arevalo, Marcus J. Bolton, Valerie Le Sage, Naiqing Ye, Colleen Furey, Hiromi Muramatsu, Mohamad-Gabriel Alameh, Norbert Pardi, Elizabeth M. Drapeau, Kaela Parkhouse, Tyler Garretson, Jeffrey S. Morris, Louise H. Moncla, Ying Okay. Tam, Steven H. Y. Fan, Seema S. Lakdawala, Drew Weissman and Scott E. Hensley, 24 November 2022, Science.
DOI: 10.1126/science.abm0271
“Mosaic RBD nanoparticles defend in opposition to problem by various sarbecoviruses in animal fashions” by Alexander A. Cohen, Neeltje van Doremalen, Allison J. Greaney, Hanne Andersen, Ankur Sharma, Tyler N. Starr, Jennifer R. Keeffe, Chengcheng Fan, Jonathan E. Schulz, Priyanthi N. P. Gnanapragasam, Leesa M. Kakutani, Anthony P. West, Greg Saturday, Yu E. Lee, Han Gao, Claudia A. Jette, Mark G. Lewis, Tiong Okay. Tan, Alain R. Townsend, Jesse D. Bloom, Vincent J. Munster and Pamela J. Bjorkman, 5 July 2022, Science.
DOI: 10.1126/science.abq0839
“An influenza hemagglutinin stem nanoparticle vaccine induces cross-group 1 neutralizing antibodies in wholesome adults” by Alicia T. Widge, Amelia R. Hofstetter, Katherine V. Houser, Seemal F. Awan, Grace L. Chen, Maria C. Burgos Florez, Nina M. Berkowitz, Floreliz Mendoza, Cynthia S. Hendel, LaSonji A. Holman, Ingelise J. Gordon, Preeti Apte, C. Jason Liang, Martin R. Gaudinski, Emily E. Coates, Larisa Strom, Diane Wycuff, Sandra Vazquez, Judy A. Stein, Jason G. Gall, William C. Adams, Kevin Carlton, Rebecca A. Gillespie, Adrian Creanga, Michelle C. Crank, Sarah F. Andrews, Mike Castro, Leonid A. Serebryannyy, Sandeep R. Narpala, Christian Hatcher, Bob C. Lin, Sarah O’Connell, Alec W. Freyn, Victoria C. Rosado, Raffael Nachbagauer, Peter Palese, Masaru Kanekiyo, Adrian B. McDermott, Richard A. Koup, Lesia Okay. Dropulic, Barney S. Graham, John R. Mascola, Julie E. Ledgerwood, on behalf of the VRC 321 examine group. , Allison Beck, Joseph Casazza, Christopher L. Case, John Misasi, Abidemi O. Ola, Karen Parker, Richard Wu, Pamela Costner, Jamie Saunders, Laura Novik, William Whalen, Xiaolin Wang, Aba Mensima Eshun, Jennifer Cunningham, Anita Arthur, Morgan Anderson, Justine Jones, Brenda Larkin, Thuy Nguyen, Sandra Sitar, Lam Le, Iris Pittman, Olga Vasilenko, Galina Yamshchikov, Ro Shauna Rothwell, Eugenia Burch, Olga Trofymenko, Sarah Plummer, Catina Evans, Cora Trelles Cartagena, Renunda Hicks, LaShawn Requilman, Pernell Williams, Carmencita Graves, Shinyi Telscher, Gabriela Albright, Jessica Bahorich, Sashikanth Banappagari, Michael Bender, Alegria T. Caringal, Juliane Carvalho, Rajoshi Chaudhuri, Mythili Chintamani, Jonathan Cooper, Jacob Demirji, Tracey Dinh, Gelu Dobrescu, Alvenne Goh, Deepika Gollapudi, Raju Gottumukkala, Daniel Gowetski, Janel Holland-Linn, Jin Sung Hong, Joe Horwitz, Vera Ivleva, Lisa Kueltzo, Nadji Lambert, Alaina LaPanse, Heather Lawlor, Kristin Leach, James Lee, Paula Lei, Yile Li, Jie (Amy) Liu, Slobodanka Manceva, Aakash Patel, Rahul Ragunathan, Lori Romaine, Erwin Rosales, Nikki Schneck, William Shadrick, Andrew Shaddeau, Sudesh Upadhyay, Karen Vickery, Xiangchun (Eric) Wang, Xin Wang, Jack Yang, Rong (Sylvie) Yang, Yanhong Yang, Yansong Yi, Weidong Zhao and Zhong Zhao, 19 April 2023, Science Translational Medication.
DOI: 10.1126/scitranslmed.ade4790
“An influenza H1 hemagglutinin stem-only immunogen elicits a broadly cross-reactive B cell response in people” by Sarah F. Andrews, Lauren Y. Cominsky, Geoffrey D. Shimberg, Rebecca A. Gillespie, Jason Gorman, Julie E. Raab, Joshua Model, Adrian Creanga, Suprabhath R. Gajjala, Sandeep Narpala, Crystal S. F. Cheung, Darcy R. Harris, Tongqing Zhou, Ingelise Gordon, LaSonji Holman, Floreliz Mendoza, Katherine V. Houser, Grace L. Chen, John R. Mascola, Barney S. Graham, Peter D. Kwong, Alicia Widge, Lesia Okay. Dropulic, Julie E. Ledgerwood, Masaru Kanekiyo, Adrian B. McDermott, 19 April 2023, Science Translational Medication.
DOI: 10.1126/scitranslmed.ade4976