What You Ought to Know:
- UltraSight, a digital health pioneer remodeling cardiac imaging by way of the ability of synthetic intelligence, introduced that it has been granted FDA clearance for its AI-powered ultrasound steerage know-how.
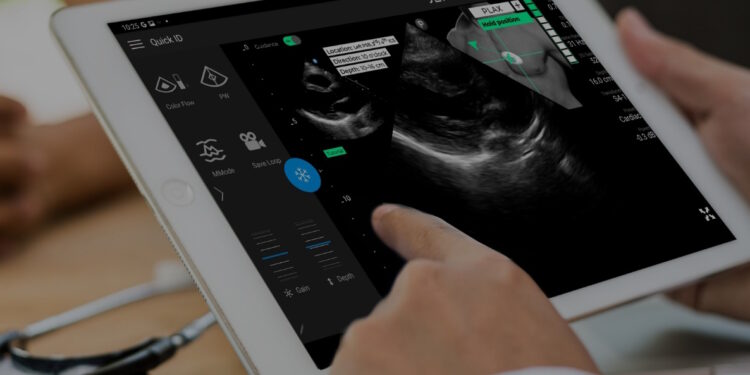
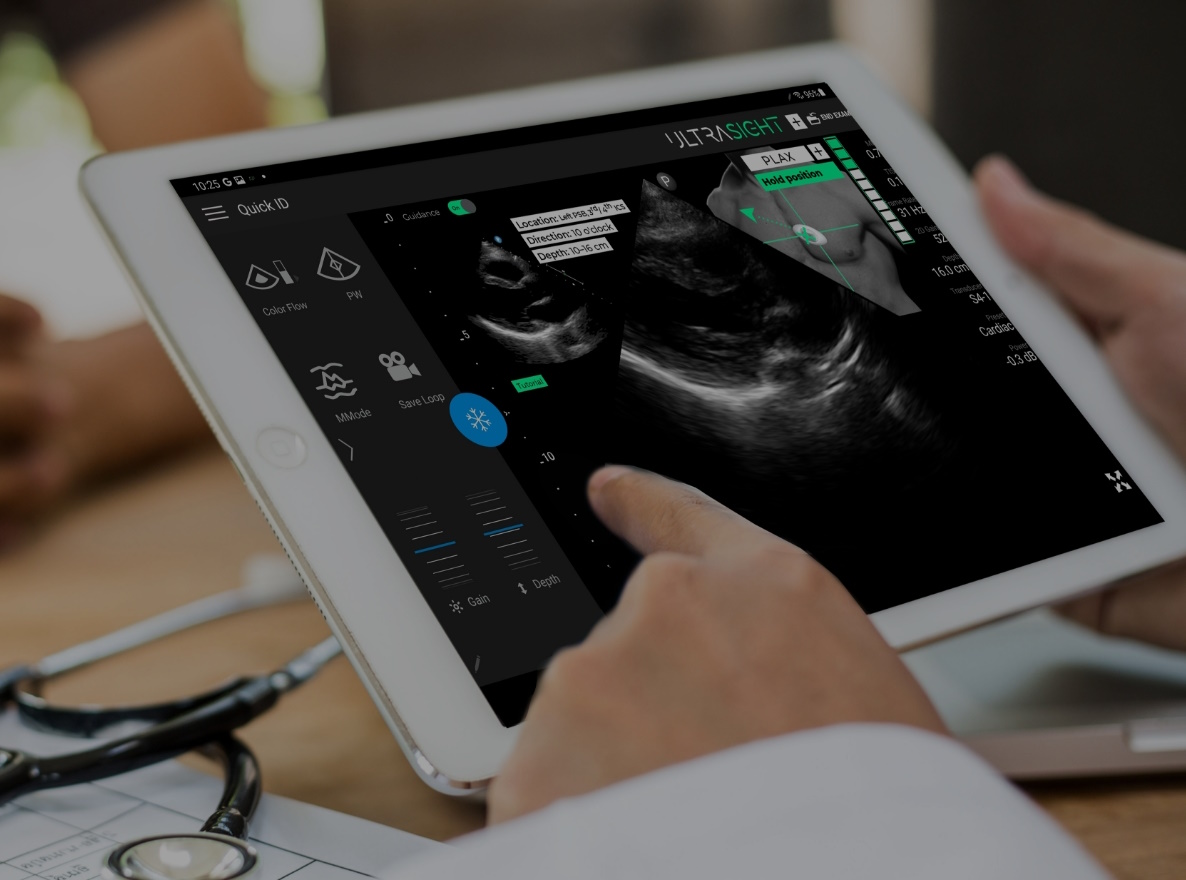
- The UltraSight real-time AI steerage software program can help medical professionals with out sonography expertise in buying cardiac ultrasound photos on the level of care in a number of settings, permitting for extra widespread detection of coronary heart illness and offering sufferers simpler entry to cardiac monitoring.
Increasing Affected person Entry to Cardiac Imaging
UltraSight’s AI Steerage software program is indicated to be used in two-dimensional transthoracic echocardiography (2D-TTE) for grownup sufferers, particularly within the acquisition of the ten commonplace views of the guts. The corporate’s submission for FDA clearance was based mostly on its landmark pivotal study which demonstrated that with real-time steerage of the ultrasound probe and suggestions on the standard of the ultrasound picture, medical professionals with out prior ultrasound expertise can purchase diagnostic high quality photos.
UltraSight’s resolution will allow hospital workers to advance affected person triage and therapy with elevated effectivity and scientific confidence. It may additionally improve entry to take care of persistent coronary heart illness sufferers by bringing cardiac ultrasound into native communities, probably growing affected person adherence to important therapies.
UltraSight’s software program is designed as an adjunct for level of care ultrasound programs and is appropriate with the Philips Lumify Ultrasound System. When paired with a appropriate machine, UltraSight’s underlying AI neural community predicts the place of the ultrasound probe relative to the guts, based mostly on the ultrasound video stream, and guides the consumer on maneuvering the probe to seize diagnostic high quality cardiac photos.