New analysis exhibits qiliqiangxin, a standard Chinese language drugs, successfully reduces hospitalizations and cardiovascular deaths in coronary heart failure sufferers with decreased ejection fraction (HFrEF). Credit score: SciTechDaily.com
Qiliqiangxin, a standard Chinese language herb mix, successfully reduces coronary heart failure hospitalizations and deaths, proving protected and useful in a large-scale medical trial.
The standard Chinese language drugs qiliqiangxin reduces hospitalization for coronary heart failure and cardiovascular dying in sufferers with coronary heart failure and a decreased ejection fraction (HFrEF), in line with late-breaking analysis introduced in a Sizzling Line session at ESC Congress 2023.[1]
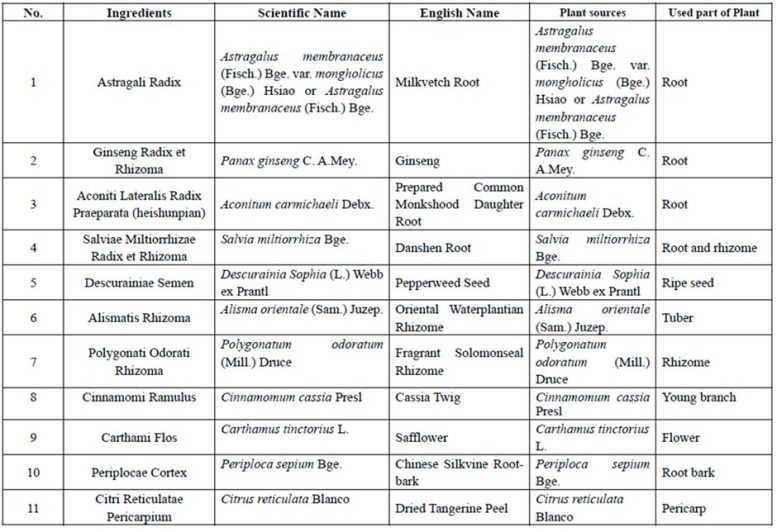
Qiliqiangxin is a standard Chinese language drugs extract obtained from 11 sorts of herbs (Desk 1).[2] In a pilot research, qiliqiangxin decreased N-terminal professional–B-type natriuretic peptide (NT-proBNP) ranges and improved coronary heart failure signs in sufferers with HFrEF when added to established coronary heart failure remedy.3 Preclinical research have additionally indicated that qiliqiangxin has useful results on attenuating myocardial fibrosis and cardiac transforming.[4-7]
The QUEST Trial: A Complete Analysis
The QUEST trial evaluated the medical efficacy and security of qiliqiangxin on main coronary heart failure outcomes in HFrEF sufferers. The trial was performed at 133 hospitals in mainland China and Hong Kong SAR of China.
The trial enrolled grownup HFrEF sufferers with a left ventricular ejection fraction of 40% or beneath and NT-proBNP of 450 pg/ml or greater who had been on a steady standardized baseline remedy routine for a minimum of two weeks previous to enrolment. Sufferers had been randomized in a 1:1 vogue to obtain qiliqiangxin (4 capsules, thrice each day) or placebo on prime of ordinary medicines for continual coronary heart failure. The first endpoint was a composite of rehospitalization for worsening coronary heart failure or cardiovascular dying.
A complete of three,110 sufferers had been included within the evaluation, with 1,555 randomized to qiliqiangxin and 1,555 randomized to placebo. The typical age was 62 years and 72.1% had been males. At baseline, the imply left ventricular ejection fraction was 32%, and the median NT-proBNP was 1730.80 pg/ml.
Important Discount in Coronary heart Failure Outcomes
Throughout a median follow-up of 18.3 months, the first endpoint occurred in 389 sufferers (25.02%) within the qiliqiangxin group and in 467 sufferers (30.03%) within the placebo group (hazard ratio [HR], 0.78; 95% confidence interval [CI], 0.68 to 0.90; p<0.001). This impact was associated to each decrease dangers of rehospitalization for worsening coronary heart failure (HR, 0.76; 95% CI, 0.64 to 0.90; p=0.002) and cardiovascular dying (HR, 0.83; 95% CI, 0.68 to 0.996; p=0.045) within the qiliqiangxin group. The impact of qiliqiangxin on the first end result was typically constant throughout prespecified subgroups together with within the subgroups outlined in line with age and NT-proBNP stage, and in sufferers with or with out angiotensin receptor/neprilysin inhibitors (ARNIs).
Secondary Findings and Security Evaluation
When it comes to secondary endpoints, the lower in serum NT-proBNP between baseline and three-month follow-up was larger within the qiliqiangxin group (-444.00 [interquartile range -1401.00 to 85.00]) than within the placebo group (-363.00 [interquartile range -1280.00 to 183.00]) (p=0.047), which was in keeping with the earlier pilot research.3[]
Evaluation of security endpoints demonstrated no important distinction in all-cause mortality, which occurred in 221 sufferers (14.21%) within the qiliqiangxin group and 262 sufferers (16.85%) within the placebo group (HR, 0.84; 95% CI, 0.70 to 1.01; p=0.058). Qiliqiangxin capsules had been well-tolerated, with no main variations between the 2 teams in hostile occasions together with gastrointestinal signs, worsening renal operate, and elevated liver enzymes.
Knowledgeable Opinion and Conclusion
Principal investigator Professor Xinli Li of the First Affiliated Hospital of Nanjing Medical College, Nanjing, China stated: “To our information, this was the primary randomized, double-blind managed trial of a standard Chinese language drugs for the remedy of continual coronary heart failure. Our findings exhibit significant medical profit with qiliqiangxin in sufferers with HFrEF, which help using qiliqiangxin as an adjunct remedy for treating coronary heart failure.”
References
- QUEST was mentioned throughout Sizzling Line 2 in room Amsterdam.
- Reference: “Research protocol for a randomized managed trial: Qiliqiangxin in coronary heart failUre: assESsment of discount in morTality (QUEST)” by Wenming Yao, Iokfai Cheang, Shengen Liao, Yanli Zhou, Fang Zhou, Dongjie Xu, Zhenhua Jia, Liping Chang, Haifeng Zhang and Xinli Li, 5 February 2020, BMC Complementary Drugs and Therapies.
DOI: 10.1186/s12906-020-2821-0 - “A Multicenter, Randomized, Double-Blind, Parallel-Group, Placebo-Managed Research of the Results of Qili Qiangxin Capsules in Sufferers With Continual Coronary heart Failure” by Xinli Li, Jian Zhang, Jun Huang, Aiqun Ma, Jiefu Yang, Weimin Li, Zonggui Wu, Chen Yao, Yuhui Zhang, Wenming Yao, Boli Zhang and Runlin Gao, 7 June 2013, Journal of the American School of Cardiology.
DOI: 10.1016/j.jacc.2013.05.035 - “Qiliqiangxin alleviates Ang II-induced CMECs apoptosis by downregulating autophagy through the ErbB2-AKT-FoxO3a axis” by Fuhai Li, Jingfeng Wang, Yu Track, Dongli Shen, Yongchao Zhao, Chaofu Li, Mingqiang Fu, Yanyan Wang, Baozheng Qi, Xueting Han, Aijun Solar, Jingmin Zhou and Junbo Ge, 27 February 2021, Life Sciences.
DOI: 10.1016/j.lfs.2021.119239 - “Conventional Chinese language drugs qiliqiangxin attenuates phenylephrine-induced cardiac hypertrophy through upregulating PPARγ and PGC-1α” by Rong-Rong Gao, Xiao-Dong Wu, Hui-Min Jiang, Yu-Jiao Zhu, Yan-Li Zhou, Hai-Feng Zhang, Wen-Ming Yao, Yong-Qin Li and Xin-Li Li, 26 April 2018, Annals of Translational Drugs.
DOI: 10.21037/atm.2018.04.14 - “Qiliqiangxin improves cardiac operate and attenuates cardiac remodelling in doxorubicin-induced coronary heart failure rats” by Xutao Solar, Guozhen Chen, Ying Xie, Deyou Jiang, Jieru Han, Fei Chen andYunjia Track, 19 Could 2023, Pharmaceutical Biology.
DOI: 10.1080/13880209.2020.1761403 - “Qiliqiangxin Modulates the Intestine Microbiota and NLRP3 Inflammasome to Defend In opposition to Ventricular Transforming in Coronary heart Failure” by Yingdong Lu, Mi Xiang, Laiyun Xin, Yang Zhang, Yuling Wang, Zihuan Shen, Li Li and Xiangning Cui, 13 April 2022, Frontiers in Pharmacology.
DOI: 10.3389/fphar.2022.905424
Funding: Nationwide Key Applied sciences R&D Program (CN, Undertaking No. 2017YFC1700500, 2017YFC1700505); Key Program of Nationwide Pure Science Basis (CN, Undertaking No. 81730106); Basic Program of Nationwide Pure Science Basis (CN, 81970339, 82270394, 82200425). Shijiazhuang Yiling Pharmaceutical Co., Ltd. (Shijazhuang, Individuals’s Republic of China) offered a part of the funding and the research drug for this analysis. All funding sources weren’t concerned within the design of the research and assortment, enrolment, statistical evaluation, interpretation of information and in writing the manuscript.
Disclosures: Prof. Xinli Li experiences receiving grant help (all grant help listed paid to the First Affiliated Hospital with Nanjing Medical College) from Novartis and China Coronary heart Failure Heart, receiving lecture charges and consulting charges from AstraZeneca, Bayer, Novartis, Roche, and Yiling.